Sacral Neuromodulation
Sacral neuromodulation is a procedure used to treat men and women with chronic urinary retention, as well as symptoms of overactive bladder (a frequent and urgent need to pass urine, with associated leakage of urine). It can also be used to treat fecal (bowel) incontinence. Clean intermittent self catheterization (CISC) or indwelling catheters in the bladder are the only other known methods for managing urinary retention. Research studies have shown cure or improvement in symptoms in up to 80% of patients with overactive bladder or urinary retention.
In this Health Topic
The sacral nerves, located near the tailbone, control the bladder and muscles related to urinary function. If the brain and sacral nerves do not communicate correctly, the bladder cannot function properly, which can cause bladder control problems. The bladder may be overactive causing urgency, or underactive causing urinary retention.
Sacral neuromodulation targets the communication problem between the brain and sacral nerves by stimulating the nerves with mild electrical pulses. Electrical stimulation is delivered to the sacral nerves through a small stimulator device that is implanted under the skin just above the buttocks. A wire carries electrical pulses from the stimulator to the sacral nerves. A hand-held programmer is used to adjust the level of stimulation and switch the device on or off. By stimulating the sacral nerves, sacral neuromodulation helps the brain and the nerves to communicate so the bladder can function properly.
The treatment involves a two-stage surgical procedure performed under local anesthesia with sedation or general anesthesia. The initial test phase, the 1st Stage, requires a 2- to 8-week assessment. This allows your doctor and you to assess your initial response with an external neuromodulator device in order to determine if a permanent device will be a good option for you.
The 1st stage procedure involves making three tiny incisions in the lower back. Through one of the incisions the permanent electrode is placed near the sacral nerve. A temporary lead is connected to the electrode, tunneled under the skin across your back where it is brought out to the opposite side. It will be connected to an external control device. You will be connected to this external device the day after your surgery.
During the test phase, you will learn how to use the stimulator to see how successful it will be in controlling your symptoms. During this phase, your doctor may ask you to keep a bladder diary to see how well the device is working. You will need to adapt your lifestyle and day-to-day work with the implant and the medical team will assess the viability of a permanent sacral neuromodulator implant. You should avoid bending, stretching, sexual intercourse, or activities that may affect the device and affect its wire connection. You should not remove any of the dressings on your back and keep the device dry at all times. As an alternative to this 1st stage, some doctors perform an office procedure called a Peripheral Nerve Evaluation under local anesthesia.
You will be taught how to connect, switch on and off and increase electrical impulses, also known as amplitude, on your stimulator the day after your 1st stage surgery. Once switched on, you may feel a pulsing, tingling, tapping, dragging or pulling sensation anywhere from your urethra (the tube leading from the bladder) to your anus (back passage).
The possible side effects of this procedure may include pain, skin irritation, infection, device problems, uncomfortable stimulation, and lead movement. The pain may radiate down the bottom of your back, buttock, and thigh to your toes. Occasionally, temporary weakness of the leg has been reported. The lead and the battery must be handled carefully. If pulled this may result in movement of the permanent electrode leading to loss of sensation or pain as described above. If this happens you might need to have the 1st stage repeated. The 1st stage procedure is reversible and can be stopped at any time by the doctor.
It is essential that you complete any bladder diaries or investigations required by your medical team following the 1st stage in order to accurately assess your response.
The 2nd stage procedure generally requires a 1- to 2 day stay in the hospital and is performed under general anesthesia. Through a small incision on your back just above the buttocks a permanent neuromodulator battery (Figure 1) is implanted in a pouch under the skin.
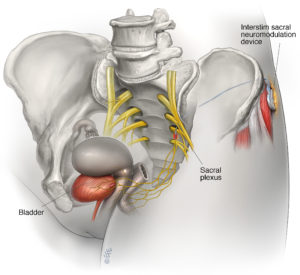
Figure 1
Your modulator will be switched on using a digital handset. No external wires will be visible. You will be shown how to use your own personal programmer which will enable you to switch the implant on and off and change the settings. At times, the settings may need fine tuning which means you will need to return to your doctor for the device to be reprogrammed. Reprogramming is usually needed because of loss of sensation. The device works best if it operates all the time, day and night. You may switch it off at any time. You should also carefully read the instruction booklet provided with the device.
The usual battery life of a modulator is more than 15 years. It may vary depending upon how your modulator is functioning. You may notice your symptoms coming back. The stimulator will need to be replaced if the battery has run down, but the electrodes do not need to be changed.
Implantation of a sacral neuromodulator is contraindicated (absolutely not allowed under any circumstances because the risk outweighs any benefits) for:
- Patients for whom 1st stage test stimulation is unsuccessful
- Patients who are unable to properly operate the system
The system may be affected by, or adversely affect, a variety of electronic medical devices or security devices (see below). Other problems include pain at the implant sites, new pain, lead migration, infection, technical or device problems, undesirable change in bowel or voiding function, and undesirable stimulation or sensations including jolting or shock sensations. Sometimes these problems require the device to be removed. Approximately one third of patients might need further surgery because of problems with the device.
Please contact your treating doctor if you need any advice. Always inform your doctors that you have a sacral neuromodulator if you are having any kind of surgery or imaging investigation. Treatments like magnetic resonance imaging (MRI), lithotripsy (for kidney stones), therapeutic ultrasound radiation therapy over the stimulator, and heart defibrillators should be avoided. MRI scans of the brain are safe with some stimulator models, and your doctor will give you information about the safety of MRI scans. If you ever have to undergo surgery, show your surgical team the sacral neuromodulation identification card. Patients who have had a neuromodulator implanted should not have certain types of diathermy (a type of energy used in surgical procedures).
You should also avoid extreme sports and diving. Mobile phones and computers do not affect the device, but do not keep your mobile device in your back pocket or close to the device. There are no restrictions on sexual intercourse.
At the airport it is advisable to avoid (if possible) going through the security screening device. Show the security personnel your sacral neuromodulation identification card and they may let you bypass the system although this is not always guaranteed. If you do have to pass through such devices it is essential that you turn the neuromodulator off. It is safe to fly with the device.
The effect of neuromodulation on pregnancy is largely unknown. Therefore, you are advised to have your device turned off by the hospital if you are planning to start a family or as soon as you know you are pregnant. Having a neuromodulator does not mean that you need to have an elective caesarean section. It is best left to the obstetrician to decide if you need one.
